Has Science Already Answered.......
Moderator: Vraith
- wayfriend
- .
- Posts: 20957
- Joined: Wed Apr 21, 2004 12:34 am
- Has thanked: 2 times
- Been thanked: 4 times
Think of it this way. Heat is like electricity -- there are things it moves through easily (conductors) and things it moves through with difficulty (insulators). Water is a conductor, like a copper wire is to electricity. Air is an insulator, like a plastic sheath around that copper wire.
Now, thermal conductivity arises (in part) from density, as has been described upthread. But my point isn't to argue that - my point is that thinking in terms of conductors and insulators makes it easy to grasp.
Cold water drains away your body temperature rapidly because water is a good thermal conductor. It's like putting a battery in a jar of copper bearings - the electricity will raidly drain out. Hot air heats your body poorly because it is a poor thermal conductor. It's like putting a battery in a jar of styrofoam peanuts.
This is why all the insulated water bottles you buy have a layer of air in them. This is why the walls of your house have foam insulation, which is really just air that is wind-proof. This is why your beer coozie is made of mostly air.
So, to get back to peter's last comment ....
Think of the temperature difference as the pressure which induces the heat to move from one place to the other. But think of the thermal conductivity as the pipe the heat goes through to get there. Water is a big wide sewer pipe, while air is a tiny plastic stirring straw. Given the same amount of pressure, heat will transfer faster through the former than the latter.
Now, the thing science has NEVER explained is: how does a cup of hot coffee which is left out on your desk get so damn cold!
[/randomness]
Now, thermal conductivity arises (in part) from density, as has been described upthread. But my point isn't to argue that - my point is that thinking in terms of conductors and insulators makes it easy to grasp.
Cold water drains away your body temperature rapidly because water is a good thermal conductor. It's like putting a battery in a jar of copper bearings - the electricity will raidly drain out. Hot air heats your body poorly because it is a poor thermal conductor. It's like putting a battery in a jar of styrofoam peanuts.
This is why all the insulated water bottles you buy have a layer of air in them. This is why the walls of your house have foam insulation, which is really just air that is wind-proof. This is why your beer coozie is made of mostly air.
So, to get back to peter's last comment ....
Think of the temperature difference as the pressure which induces the heat to move from one place to the other. But think of the thermal conductivity as the pipe the heat goes through to get there. Water is a big wide sewer pipe, while air is a tiny plastic stirring straw. Given the same amount of pressure, heat will transfer faster through the former than the latter.
Now, the thing science has NEVER explained is: how does a cup of hot coffee which is left out on your desk get so damn cold!
[/randomness]
.
- Skyweir
- Lord of Light
- Posts: 25357
- Joined: Sat Mar 16, 2002 6:27 am
- Location: Australia
- Has thanked: 2 times
- Been thanked: 18 times
Brilliant explanation.. even I can grasp that. I just love, love, love your combined and contributory intellect. Such spectacular genius.
I get so very excited about yalls cleverness .. just love it. I especially love it when I can understand an explanation too




keep smiling
'Smoke me a kipper .. I'll be back for breakfast!'

EZBoard SURVIVOR
- peter
- The Gap Into Spam
- Posts: 11561
- Joined: Tue Aug 25, 2009 10:08 am
- Location: Another time. Another place.
- Been thanked: 6 times
Now in the case of immersing yourself in a bath of ether ........
,........... I'd recommend that you don't lie back and light up a cigarette!!!!!
(Which reminds me of the chap who earned himself a Darwin Award when he decided to spark up a joint inside an oxygen tent with the predictably disastrous consequences in terms of his very quickly over life.)
The truth is a Lion and does not need protection. Once free it will look after itself.
....and the glory of the world becomes less than it was....
'Have we not served you well'
'Of course - you know you have.'
'Then let it end.'
We are the Bloodguard
....and the glory of the world becomes less than it was....
'Have we not served you well'
'Of course - you know you have.'
'Then let it end.'
We are the Bloodguard
- wayfriend
- .
- Posts: 20957
- Joined: Wed Apr 21, 2004 12:34 am
- Has thanked: 2 times
- Been thanked: 4 times
In my opinion, thermal capacity doesn't come into play because you are walking in a near infinite amount of air, or swimming in a near infinite amount of water. So there is a near infinite amount of capacity. If you're talking about a guy in a bathtub, then ok capacity matters.
Also, it's not like the tub of water gets full and accepts no more heat. Temperature ALWAYS evens out. All it changes is what temperature it will be when it evens out.
Also, it's not like the tub of water gets full and accepts no more heat. Temperature ALWAYS evens out. All it changes is what temperature it will be when it evens out.
.
- peter
- The Gap Into Spam
- Posts: 11561
- Joined: Tue Aug 25, 2009 10:08 am
- Location: Another time. Another place.
- Been thanked: 6 times
Absolutely fair points all round Wayfriend - but surely the hugely larger amount of available heat-energy per unit contact area in the case of water is bound to be a factor here?
(And getting really abstruse about the thing you're now down to the point of considering the effects atomic/molecular movement on the situation; in the case of heat-energy in a solid recording a temperature of 50 C, that energy is going to be be locked {as it were} into vibrational energy of individual elements (small e) held in situ within the framework of the lattice they form a part of. True also to a lesser extent with liquids, it would only be in the case of gases that the individual atoms/molecules would have the freedom to be flying about bashing into the contact interface with their full kinetic potential (as it were). On this thinking one might conjecture, if one had no empirical experience to the contrary, that a gas skin interface would undergo much more heat energy transfer from one side to the other (gas temp at 50 degrees C) than the water-skin interface at the same temp......but of course experience tells us it is not so. Hmmm.....)
I refuse absolutely to 'google' an answer to this. Firstly, I'm not at all sure that this is as well understood as on first thinking it might seem to be (something like the "why is the sky blue" question). Secondly it seems to me to be a problem that lends itself beautifully to the idea that solutions can be arrived at by nothing other than pure ratiocination - given at least a modicum of knowledge in the physical/chemical sciences - and if a solution to it is not possible to be reached in this manner (ie without recourse to experiment) in this case - I wonder if it would ever be so anywhere?
Does that idea still even exist - that knowledge can be arrived at by thought without the additional input of measurement - at all anymore?
(And getting really abstruse about the thing you're now down to the point of considering the effects atomic/molecular movement on the situation; in the case of heat-energy in a solid recording a temperature of 50 C, that energy is going to be be locked {as it were} into vibrational energy of individual elements (small e) held in situ within the framework of the lattice they form a part of. True also to a lesser extent with liquids, it would only be in the case of gases that the individual atoms/molecules would have the freedom to be flying about bashing into the contact interface with their full kinetic potential (as it were). On this thinking one might conjecture, if one had no empirical experience to the contrary, that a gas skin interface would undergo much more heat energy transfer from one side to the other (gas temp at 50 degrees C) than the water-skin interface at the same temp......but of course experience tells us it is not so. Hmmm.....)
I refuse absolutely to 'google' an answer to this. Firstly, I'm not at all sure that this is as well understood as on first thinking it might seem to be (something like the "why is the sky blue" question). Secondly it seems to me to be a problem that lends itself beautifully to the idea that solutions can be arrived at by nothing other than pure ratiocination - given at least a modicum of knowledge in the physical/chemical sciences - and if a solution to it is not possible to be reached in this manner (ie without recourse to experiment) in this case - I wonder if it would ever be so anywhere?
Does that idea still even exist - that knowledge can be arrived at by thought without the additional input of measurement - at all anymore?
The truth is a Lion and does not need protection. Once free it will look after itself.
....and the glory of the world becomes less than it was....
'Have we not served you well'
'Of course - you know you have.'
'Then let it end.'
We are the Bloodguard
....and the glory of the world becomes less than it was....
'Have we not served you well'
'Of course - you know you have.'
'Then let it end.'
We are the Bloodguard
- wayfriend
- .
- Posts: 20957
- Joined: Wed Apr 21, 2004 12:34 am
- Has thanked: 2 times
- Been thanked: 4 times
The water contacting your body absorbs heat from you but then it passes the heat on to the water next to it, ad infinitum. It's infinite regardless of the medium.peter wrote: but surely the hugely larger amount of available heat-energy per unit contact area in the case of water is bound to be a factor here?
.
- Vraith
- The Gap Into Spam
- Posts: 10621
- Joined: Fri Nov 21, 2008 8:03 pm
- Location: everywhere, all the time
In any normal situation, the infinity doesn't matter...it's much more local than that.wayfriend wrote:The water contacting your body absorbs heat from you but then it passes the heat on to the water next to it, ad infinitum. It's infinite regardless of the medium.peter wrote: but surely the hugely larger amount of available heat-energy per unit contact area in the case of water is bound to be a factor here?
One must remember the human body is not neutral...it is a waste-heat generating body that MUST be cooled. It is ALSO a system that MUST be fed to generate that heat...the generator REQUIRES a specific, narrow temperature in order to continue working.
Because of heat transfer vs. heat production, balanced with temperature requirement, as long as you have enough food and water to PRODUCE, 50F [10C] air might make you chilly, but probably won't kill you. The same temp water surroundings almost certainly will eventually. WITHOUT food and water, air that temp won't really start hurting until you are already also dying of hunger/thirst. Water will fuck you up much sooner.
For a range, that relationship inverts....again, assuming you have enough food and water.
In case someone's doubting, note this: the perfect temperatures for running are [depending on humidity] well below 60 [15c]. For water sports MINIMUM upper seventies [25c.]
Then it gets fuckity for a bit. At 100F[36ishc] It depends on what you're doing, and how much water you have to drink. [AND you MUST be in shade/darkness for air, starting well below 100...cuz sun will fuck you the hell up--you're gaining WAY more heat than ambient temperature]
Above 115-ish, I have no idea what happens...I never saw anyone studied long-exposure except entirely in air of various humitidies. [[higher humitidy=more hurt IF you have plenty of water to drink in dry conditions to allow natural cooling to happen]...but I'd wager that unless you are lying around naked talking Swedish or Finnish or Icelandcalese in your lodge, both will lay you low very quickly...until the next temp break....
mid 100's somewhere, water will start cooking you, while air won't [for a while, if you are well-hydrated]
People fairly regularly do dry sauna, for short times, 190+[90+], and some higher. You CAN burn at 130+/- in water, probably will at 150 almost immediately, at 190, you'll be lucky to live and certainly permanently scarred everywhere pretty much instantaneously [up-side: if you live, most of your surface nerve endings will be dead, so you can impress your friends at the bar by slicing, poking, and burning yourself without screaming for the rest of your life. Of course, if your nether regions were involved in the immersion, most sex, and definitely any orgasms/joy, no matter your initial equipment, will be history]
[spoiler]Sig-man, Libtard, Stupid piece of shit. change your text color to brown. Mr. Reliable, bullshit-slinging liarFucker-user.[/spoiler]
the difference between evidence and sources: whether they come from the horse's mouth or a horse's ass.
"Most people are other people. Their thoughts are someone else's opinions, their lives a mimicry, their passions a quotation."
the hyperbole is a beauty...for we are then allowed to say a little more than the truth...and language is more efficient when it goes beyond reality than when it stops short of it.
the difference between evidence and sources: whether they come from the horse's mouth or a horse's ass.
"Most people are other people. Their thoughts are someone else's opinions, their lives a mimicry, their passions a quotation."
the hyperbole is a beauty...for we are then allowed to say a little more than the truth...and language is more efficient when it goes beyond reality than when it stops short of it.
- FindailsCrispyPancakes
- <i>Elohim</i>
- Posts: 207
- Joined: Fri Jun 07, 2019 10:47 pm
Agreed, temperature does always even out when systems interact, as heat always flows from a hot system to a cold one. I would add the caveat that the time taken to achieve thermal equilibrium depends not only upon the mass/energy, volume and thermal conductivity of the systems in question, but upon issues of limiting boundaries/localisation.
When considering heat exchange, regardless of the size of the surrounding medium (which could well be very large but not infinite), the main factors are the localised temperatures of system A (dependent upon the mass/energy density of the object, in this case a person) and system B (dependent on the mass/energy density of the surrounding medium, which in this case is air/water) and the thermal conductivity of these systems.
In the case of a dynamic exchange such as a living person in a tub of water, thermal equilibrium between systems A & B can only be achieved if that person is willing to stop eating/drinking/moving, die and decompose down to their constituent atoms in the same time it takes the bath water to evaporate away. You'd need a hell of a tub. It's much easier to admit that these problems make little sense without sensible limiting boundaries and declare that the natural tendency is for the systems to approach thermal equilibrium.
The object may be surrounded by a vast medium but the heat exchange between the object and the surrounding medium will be at its maximum in the immediate vicinity of the object. The measured amount of heat exchange is dependent upon where measurements are taken from within the surrounding medium.
Prior to thermal equilibrium (without sensible limiting boundaries the words 'prior to thermal equilibrium' would mean you'd be in the bath until the end of time), the heat exchange between the object and the medium will seem to be far lower when measured at a greater distance from the object than when measured in close proximity to the object, so (as with all physics problems involving objects situated within large scale mediums) the localisation/measurement issues must be considered.
For example, the air temperature in sunny Australia will have little effect on the localised heat exchange between object and medium if your object is surrounded by the freezing air of Iceland during the darkness of an Arctic winter. It's analagous to a distant sound getting quieter until it's indistinguishable from the background noise.
Those are the kind of limiting factors that need to be programmed into the framework of any calculations for a problem like this. Without setting sensible limits (ultimately it's a question of quantization) there's no way to avoid infinities or unrealistic results.
There's a few other things that would affect the results:
1) The mass density of water is over 800 times that of air.
2) There's not much difference between the density of the human body (which is roughly 60% water anyway) and the density of water.
3) The thermal conductivity of the human body is approximately one third of the thermal conductivity of water at body temperature (this varies dependent on water purity).
4) The thermal conductivity of water at body temperature is over 20 times greater than that of air at body temperature (this varies dependent on water purity).
In relation to the 50 degrees centigrade bath (ouch) question:
The thermal conductivity of water at 50 degrees centigrade is over 30 times that of air at 50 degrees centigrade (this varies dependent on water purity).
When considering heat exchange, regardless of the size of the surrounding medium (which could well be very large but not infinite), the main factors are the localised temperatures of system A (dependent upon the mass/energy density of the object, in this case a person) and system B (dependent on the mass/energy density of the surrounding medium, which in this case is air/water) and the thermal conductivity of these systems.
In the case of a dynamic exchange such as a living person in a tub of water, thermal equilibrium between systems A & B can only be achieved if that person is willing to stop eating/drinking/moving, die and decompose down to their constituent atoms in the same time it takes the bath water to evaporate away. You'd need a hell of a tub. It's much easier to admit that these problems make little sense without sensible limiting boundaries and declare that the natural tendency is for the systems to approach thermal equilibrium.
The object may be surrounded by a vast medium but the heat exchange between the object and the surrounding medium will be at its maximum in the immediate vicinity of the object. The measured amount of heat exchange is dependent upon where measurements are taken from within the surrounding medium.
Prior to thermal equilibrium (without sensible limiting boundaries the words 'prior to thermal equilibrium' would mean you'd be in the bath until the end of time), the heat exchange between the object and the medium will seem to be far lower when measured at a greater distance from the object than when measured in close proximity to the object, so (as with all physics problems involving objects situated within large scale mediums) the localisation/measurement issues must be considered.
For example, the air temperature in sunny Australia will have little effect on the localised heat exchange between object and medium if your object is surrounded by the freezing air of Iceland during the darkness of an Arctic winter. It's analagous to a distant sound getting quieter until it's indistinguishable from the background noise.
Those are the kind of limiting factors that need to be programmed into the framework of any calculations for a problem like this. Without setting sensible limits (ultimately it's a question of quantization) there's no way to avoid infinities or unrealistic results.
There's a few other things that would affect the results:
1) The mass density of water is over 800 times that of air.
2) There's not much difference between the density of the human body (which is roughly 60% water anyway) and the density of water.
3) The thermal conductivity of the human body is approximately one third of the thermal conductivity of water at body temperature (this varies dependent on water purity).
4) The thermal conductivity of water at body temperature is over 20 times greater than that of air at body temperature (this varies dependent on water purity).
In relation to the 50 degrees centigrade bath (ouch) question:
The thermal conductivity of water at 50 degrees centigrade is over 30 times that of air at 50 degrees centigrade (this varies dependent on water purity).
- FindailsCrispyPancakes
- <i>Elohim</i>
- Posts: 207
- Joined: Fri Jun 07, 2019 10:47 pm
Findail's utterly trivial hierarchy of nothings
As a bit of a jape, on the something/nothing conundrum... how big is the nothing in question?
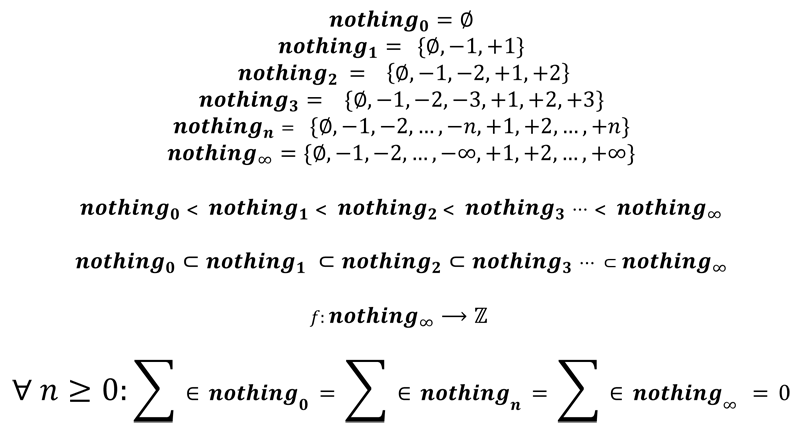
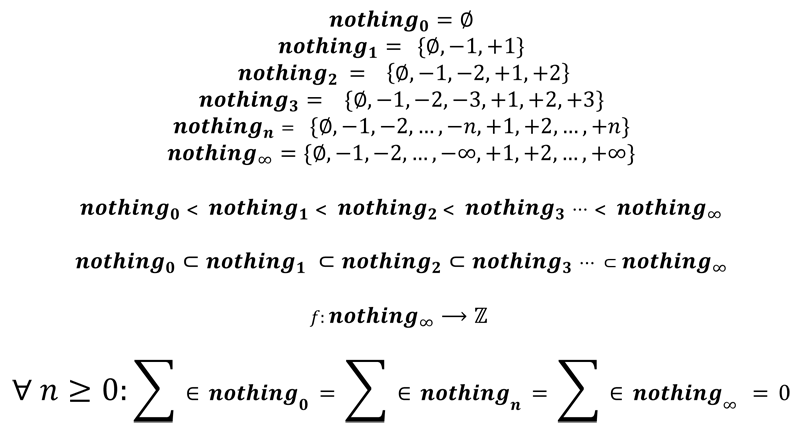
- Vraith
- The Gap Into Spam
- Posts: 10621
- Joined: Fri Nov 21, 2008 8:03 pm
- Location: everywhere, all the time
Heh...hey there, Failed Cakes...
That's in the same family [though yours is for math-savvy, mine is for math-stupified] as [a true story I think I've posted before]...
Me and my best bud in math class [roughly, with other stuff excluded]:
Teacher: So three factorial is written 3!
Us: nah, that's THREE!!!
What we wrote on board for extra credit assignment:
0!=1
1!=1
0!=1!
divide both sides by !
0=1.
We did a lot of that shit.
[[[to be fair, my friend was a year older and had already taken-and failed- the class, so it wasn't pure improv.
I don't want anyone to think he was dumb, though, even people who don't know him and never will--- he took the class, which was AP and a Regents [in NYS high-school, regents was a program that had a state-wide exam. The classes and tests were supposedly more demanding, taking them had advantages for admissions to SUNY schools, and a minimal "scholarship" if you took enough of them, in enough subjects, with high enough scores...regents still exists, it's a bit different now---anyway, first time he took the class was with a teacher who had 25 or 30 students. EVERYONE BUT TWO who took her class failed the damn test. I think that's accurate. It might have been one or ...heh...THREE!!! What I KNOW is accurate is the same year she taught the preceding regents math class as well. All THOSE students but one...me...failed that test as well. Good news: she got demoted to remedial. She would have been fired except our school was in a tiny town with shitty salaries and asshole school board, and NO ONE WANTED TO WORK THERE]]]
There was no reason other than my mood, time, and the fact i haven't talked to anyone today for that story/comment.
That's in the same family [though yours is for math-savvy, mine is for math-stupified] as [a true story I think I've posted before]...
Me and my best bud in math class [roughly, with other stuff excluded]:
Teacher: So three factorial is written 3!
Us: nah, that's THREE!!!
What we wrote on board for extra credit assignment:
0!=1
1!=1
0!=1!
divide both sides by !
0=1.
We did a lot of that shit.
[[[to be fair, my friend was a year older and had already taken-and failed- the class, so it wasn't pure improv.
I don't want anyone to think he was dumb, though, even people who don't know him and never will--- he took the class, which was AP and a Regents [in NYS high-school, regents was a program that had a state-wide exam. The classes and tests were supposedly more demanding, taking them had advantages for admissions to SUNY schools, and a minimal "scholarship" if you took enough of them, in enough subjects, with high enough scores...regents still exists, it's a bit different now---anyway, first time he took the class was with a teacher who had 25 or 30 students. EVERYONE BUT TWO who took her class failed the damn test. I think that's accurate. It might have been one or ...heh...THREE!!! What I KNOW is accurate is the same year she taught the preceding regents math class as well. All THOSE students but one...me...failed that test as well. Good news: she got demoted to remedial. She would have been fired except our school was in a tiny town with shitty salaries and asshole school board, and NO ONE WANTED TO WORK THERE]]]
There was no reason other than my mood, time, and the fact i haven't talked to anyone today for that story/comment.
[spoiler]Sig-man, Libtard, Stupid piece of shit. change your text color to brown. Mr. Reliable, bullshit-slinging liarFucker-user.[/spoiler]
the difference between evidence and sources: whether they come from the horse's mouth or a horse's ass.
"Most people are other people. Their thoughts are someone else's opinions, their lives a mimicry, their passions a quotation."
the hyperbole is a beauty...for we are then allowed to say a little more than the truth...and language is more efficient when it goes beyond reality than when it stops short of it.
the difference between evidence and sources: whether they come from the horse's mouth or a horse's ass.
"Most people are other people. Their thoughts are someone else's opinions, their lives a mimicry, their passions a quotation."
the hyperbole is a beauty...for we are then allowed to say a little more than the truth...and language is more efficient when it goes beyond reality than when it stops short of it.
- Hashi Lebwohl
- The Gap Into Spam
- Posts: 19576
- Joined: Mon Jul 06, 2009 7:38 pm
- Vraith
- The Gap Into Spam
- Posts: 10621
- Joined: Fri Nov 21, 2008 8:03 pm
- Location: everywhere, all the time
Of course, the way that I/we did it is totally not math, but can trick people with mathiness.Hashi Lebwohl wrote:This is actually a true statement if you define a group where the additive identity and the multiplicative identity are the same element.Vraith wrote:0=1
Mathematics is much stranger than many think. I run across things all the time that I tend to take people at their word about without going into the deep proof. [depending on their position in the world].
Like I'm pretty sure I saw a thing a year or so ago that was something like the sum of all numbers is -1/12 or something? [[I'm not sure that's the right number...but it was a wholly unexpected number, almost positive it was a fraction...pretty sure it was summation, not sums, same but different, which I definitely don't need to tell you.
[spoiler]Sig-man, Libtard, Stupid piece of shit. change your text color to brown. Mr. Reliable, bullshit-slinging liarFucker-user.[/spoiler]
the difference between evidence and sources: whether they come from the horse's mouth or a horse's ass.
"Most people are other people. Their thoughts are someone else's opinions, their lives a mimicry, their passions a quotation."
the hyperbole is a beauty...for we are then allowed to say a little more than the truth...and language is more efficient when it goes beyond reality than when it stops short of it.
the difference between evidence and sources: whether they come from the horse's mouth or a horse's ass.
"Most people are other people. Their thoughts are someone else's opinions, their lives a mimicry, their passions a quotation."
the hyperbole is a beauty...for we are then allowed to say a little more than the truth...and language is more efficient when it goes beyond reality than when it stops short of it.
- Gaius Octavius
- The Gap Into Spam
- Posts: 3331
- Joined: Sun Jan 07, 2018 8:32 pm
- FindailsCrispyPancakes
- <i>Elohim</i>
- Posts: 207
- Joined: Fri Jun 07, 2019 10:47 pm
Skyweir wrote:
Crispy Pants
How nice to see you in these parts
How nice to see you too Sky
Oh, I like it.Vraith wrote:Teacher: So three factorial is written 3!
Us: nah, that's THREE!!!
What we wrote on board for extra credit assignment:
0!=1
1!=1
0!=1!
divide both sides by !
0=1.
We did a lot of that shit.
Hashi Lebwohl wrote:This is actually a true statement if you define a group where the additive identity and the multiplicative identity are the same element.Vraith wrote:0=1
Great stuff. I was just fooling around on the word processor while I waited for the computer to do work!Lazy Luke wrote:Only when an element is from a Lie group.
"And the earth was without form, and void." Genesis (1:2) = 0!
Edits: Missing closing color bracket for text!
Last edited by FindailsCrispyPancakes on Sun Jul 28, 2019 9:32 am, edited 1 time in total.
EARTH:



Not even remotely harmless

Not even remotely harmless
- FindailsCrispyPancakes
- <i>Elohim</i>
- Posts: 207
- Joined: Fri Jun 07, 2019 10:47 pm


 +
+